Per-Olof Hasselgren • Scientist
Basic Research
The main focus of Dr. Hasselgren’s basic research during the last several decades has been the molecular regulation of protein degradation in skeletal muscle during conditions characterized by muscle wasting. Those conditions include cancer, injury, sepsis, high age, and malnutrition and are associated with loss of muscle mass and strength.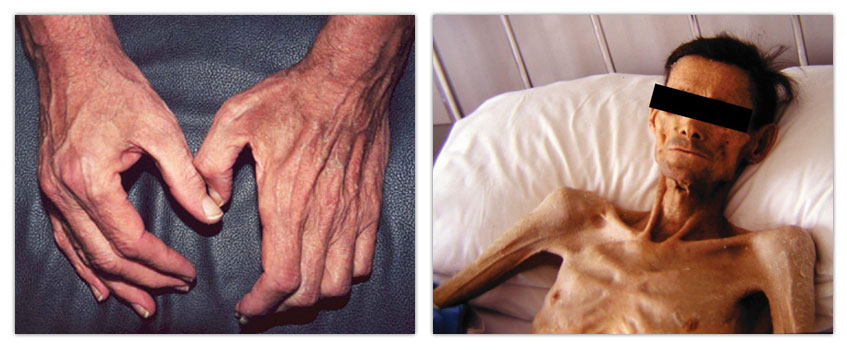
High age often results in loss of muscle mass (sarcopenia) as illustrated in the left panel. Cancer is also often associated with loss of muscle mass (cancer cachexia) resulting in pronounced weakness and ultimately in death (right panel). Other conditions resulting in muscle wasting include sepsis, severe injury, and bed rest.[/caption] Research in Dr. Hasselgren’s laboratory has revealed that the most important mechanism of loss of muscle mass in these conditions is an increase in the breakdown of muscle proteins, in particular myofibrillar proteins, although reduced synthesis of muscle proteins contributes as well. Dr. Hasselgren’s laboratory has performed pioneering research into the role of ubiquitin-proteasome-dependent protein breakdown during sepsis-induced muscle wasting since the mid-1980s. In more recent research, his laboratory was the first to report evidence that acetylation of both substrate proteins and of molecules involved in the regulation of muscle proteolysis plays important roles in the regulation of muscle proteolysis.
Research performed by Dr. Hasselgren has shown that the increased breakdown of muscle proteins during sepsis is regulated by multiple mediators, intracellular signaling pathways, transcription factors and gene regulation. An important consequence of these mechanisms is increased ubiquitination of proteins, making them susceptible to proteasome-dependent degradation. The ubiquitination of muscle proteins is regulated by multiple mechanisms, including the expression and activity of the muscle specific ubiquitin ligases atrogin-1 and MuRF1.
In addition, post-translational modifications of muscle proteins may make them more prone to undergo ubiquitination and degradation. Such modifications include phosphorylation and acetylation. Post-translational modifications may also alter the expression and activity of atrogin-1, MuRF1 and other enzymes involved in the regulation of muscle protein degradadtion. Some of the mechanisms involved in muscle wasting during sepsis, after trauma and in cancer are summarized below.
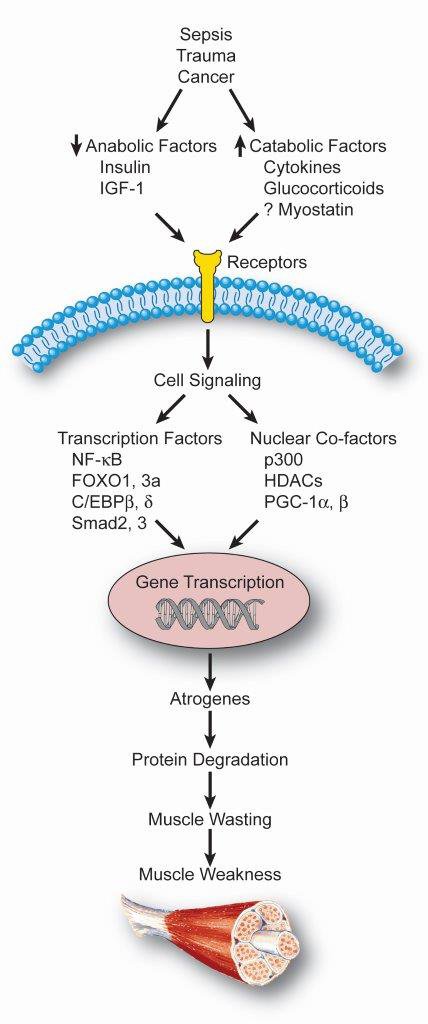
Muscle wasting induced by sepsis, trauma, and cancer, is regulated by multiple factors, including changes in the levels of anabolic and catabolic mediators, intracellular signaling pathways, transcription factors and nuclear co-factors. These factors work in concert to increase the expression and activity of the atrogenes atrogin-1 and MuRF1 resulting in ubiquitination and proteasome-dependent degradation of muscle proteins. The loss of muscle mass results in weakness with significant clinical consequences. (Reprinted from Clin Rev Clin Lab Sci 48: 71-86, 2011)
In addition to research in the field of muscle wasting during sepsis and other catabolic conditions, Dr. Hasselgren’s research laboratory has contributed important information about the regulation of protein metabolism in the liver and intestinal mucosa during these conditions. In contrast to the situation in skeletal muscle, those studies have provided evidence that proteins are preserved in the liver (at least in part reflecting increased synthesis of acute phase proteins) and gut mucosa (in part reflecting increased turnover of enterocytes). Indeed, evidence suggest that there is an interaction between these tissues with the breakdown of muscle proteins providing amino acids for the stimulated protein synthesis in liver and intestine.
Clinical Research
In addition to basic research, Dr. Hasselgren has published several clinical papers related to surgical site infections and breast and endocrine surgery. Important contributions in the field of breast surgery include papers describing the role of needle localization for the surgical removal of non-palpable breast tumors. In addition, the management of such tumors that are missed during attempted image-guided excision has also been reported by Dr. Hasselgren.
Reflecting Dr. Hasselgren’s main clinical interest, he has authored several papers in the field of endocrine surgery. In previous work, the possibility to treat hyperthyroid patients with a beta-blocker alone in preparation for thyroidectomy was investigated. Other studies examined the influence of beta-blockers on protein metabolism in skeletal muscle and in the thyroid gland in patients with hyperthyroidism. A prospective database in the Section of Endocrine Surgery presently containing clinical information for more than 4,000 patients is an important source of data supporting the clinical research. Based on such information, Dr. Hasselgren and his group have published papers related to thyroid and parathyroid surgery. For example, the experience with an unusual type of thyroid tumor (hyalinizing trabecular adenoma) was reported previously. A current research project is focused on one of the most common subtypes of papillary thyroid cancer, the follicular variant of papillary thyroid cancer.
In recent papers related to parathyroid surgery, Dr. Hasselgren reported on the use of intraoperative measurement of parathyroid hormone levels and the surgical approach when the intraoperative parathyroid hormone level does not decrease appropriately after removal of diseased parathyroid gland(s). In a recent paper, the correlation between the size of parathyroid tumors and the risk of malignancy was studied. The study showed that although the risk of cancer is increased in large parathyroid tumors (>2 g), the risk for cancer is still quite small even in large parathyroid tumors.
In other clinical research, Dr. Hasselgren collaborates with endocrinologists at the Beth Israel Deaconess Medical Center. In one of those projects, the presence of brown fat in the neck of patients undergoing thyroid surgery and its regulation by thyroid hormones were examined. Some of the results from those studies were published recently in Nature Medicine. The study has important clinical implications suggesting a potential role of thyroid hormones in the treatment of obesity. In another project performed in collaboration with endocrinologists at the Beth Israel Deaconess Medical Center, the regulation of muscle metabolism by adiponectin in patients with obesity was examined.